
 Home > Business Units
Home > Business Units
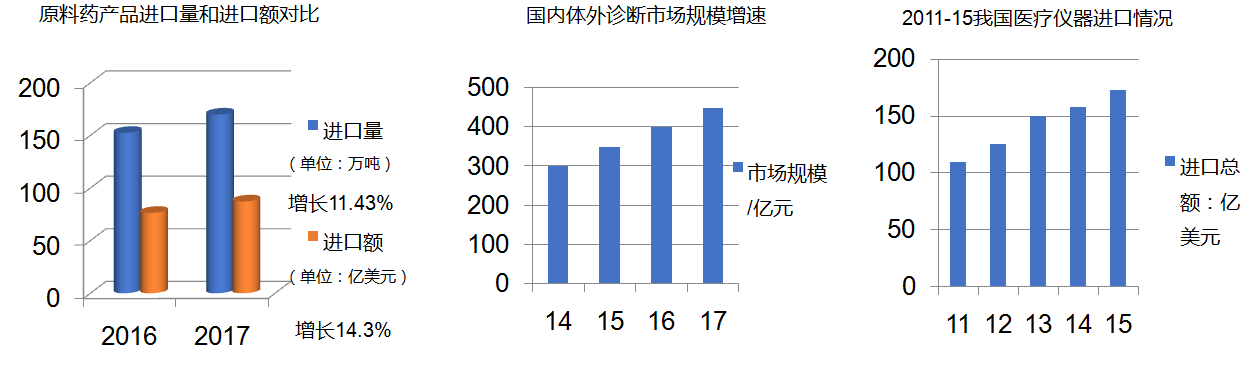
Compared with developed countries, China's per capita medical and health expenditure is still at a low level, and the domestic medical industry still needs a large number of imported medical products with advanced technology.
In recent years, the import scale of APIs, medical devices, high-value consumables and in vitro diagnostic reagents has increased steadily, especially the annual growth rate of in vitro diagnostic reagents is more than 20%.
Difficult and complicated
Muti Links
Import involves compliance, examination and approval, overseas transportation, customs clearance and domestic distribution. It needs to contact many third parties and links.
Strict Approval
For class II and class III medical devices, the import approval of IVD is strict and the approval documents are cumbersome.
Hard Temperature Control
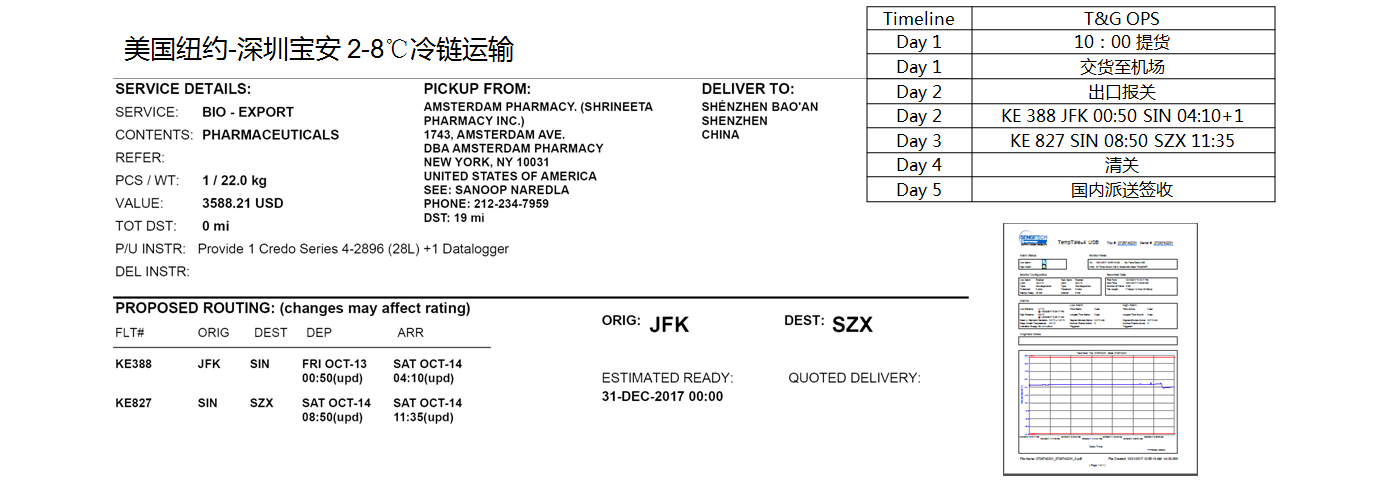
Most of the imported medical products have strict temperature requirements, so it is necessary to strictly control the temperature in the whole transportation process.
Efficient
From overseas pick up to domestic customs clearance to delivery, it requires 48-72 hours to complete, which puts forward high requirements for effictive of the whole chain.
Various Documents
The national supervision of biological and medical products is the largest of all products, and the information required for declaration is complicated and huge.
Strict government supervision
The imported biological and medical products must be sampled and inspected, and the health certificate shall be issued after passing the inspection, which are necessary before products can be sale to consumer.






 |
About Us
MORE
|
 (86)21-5308 8855 (86)21-5308 8855Room 20A-C,No.668,East Beijing Road,East Building,ShanghaI,China |